Ionic Metallic And Covalent Bonds Venn Diagram

Attraction is between the.
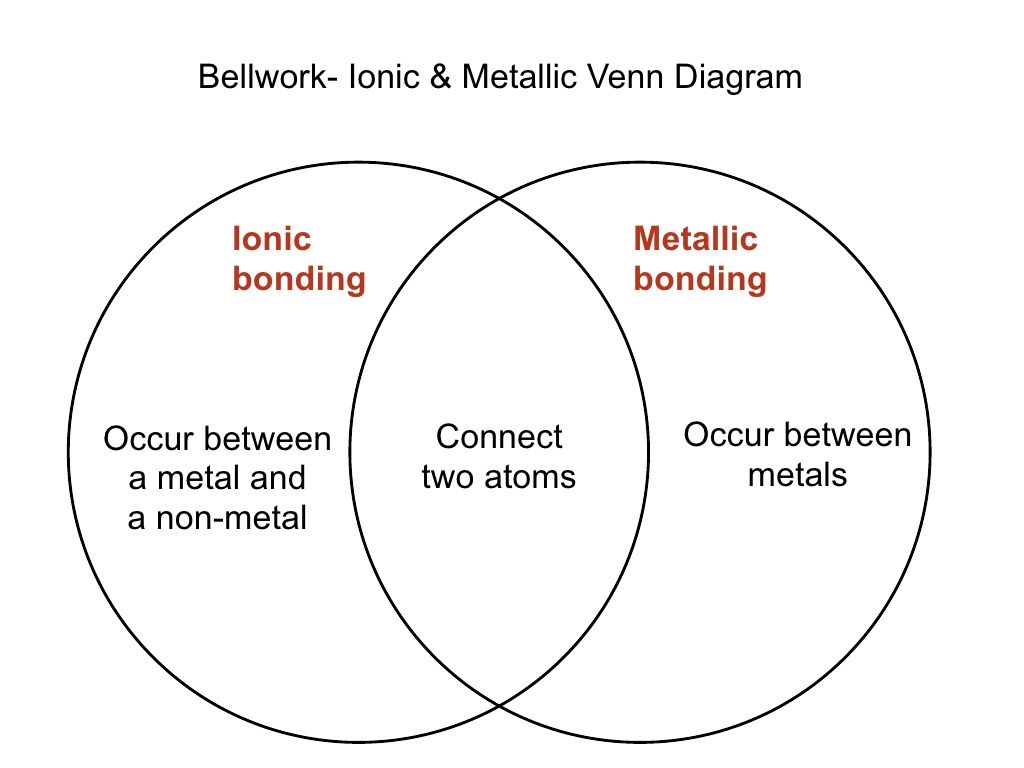
Ionic metallic and covalent bonds venn diagram. One side has the characteristic. After the introduction of quantum mechanics and the electrons the idea of the chemical bonding was put forth during the 20th century. All i need to do is make a venn diagram comparing ionic covalent metallic bonds. Day 6 venn diagram haber process.
The other side has the bond compound type shop the black friday sale. You can edit this venn diagram using creately diagramming tool and include in your report presentation website. Ionic review and mineral projectlesson 6. I already know that ionic is a metal and nonmetal covalent is 2 nonmetals and metallic is 2 metals.
Characteristics of ionic metallic and covalent bonds and compounds. Ionic and covalent bonding involves different number of atoms coming together and forming more complex structures. Start studying chemical bonding venn diagram. Metal and a non metal.
The main reason for the bonding is the decrease of the energy of the atoms because the bodies with lower energy are more stable. Primary bonds are covalent metallic and ionic bonds whereas secondary bonds are the dipole dipole interactions hydrogen bonds etc. Valence shell electron pair repulsion theory vsepr lesson 9. Get 50 off quizlet plus through monday learn more.
Ionic covalent and metallic bonding. Warm up page on your table. Metallic and covalent bonds formation and naminglesson 7. Learn vocabulary terms and more with flashcards games and other study tools.
Copy of compare contrast ionic covalent metallic bonds you can edit this template and create your own diagram creately diagrams can be exported and added to word ppt powerpoint excel visio or any other document. Day 8 metallic bonding properties of substances reading and graphic organizer. I have a 73 in chemistry right now and in order to raise it i need to do a project thats due tomorrow. Venn diagram of ionic and covalent bonding.
Ionic covalent and metallic bondslesson 4. Those are already in the venn diagram but i don t know what else to put. Metallic bonding properties of substances reading. The formed compounds have different properties from the bonding sources.
With the discussion on the chemical bonding one can get the knowledge of.